Diabetic retinopathy (DR) is the leading causes of blindness and visual impairment worldwide. One in four Europeans over the age of 60 is affected by AMD, according to the report by EURETINA (European Society of Retina Specialist).
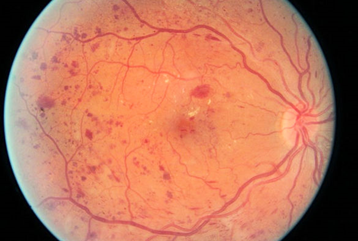
Yet, studies reported that 90% of the cases could be prevented through early detection and treatment. Given the rising incidence of the cases, it’s challenging to manually analyse the images and training new personnel is also a lengthy procedure and requires acquisition of expertise. Deep learning (LeCun et al., 2015) (DL) a family of machine learning (ML) techniques, has garnered attention recently because of its ability to learn the most predictive features from images, given a large dataset of labelled examples, without specifying rules or features explicitly. DL has been extensively used over the last several years for many automatic classification tasks, specifically, for the case of image classification. In the past, many DL classifiers for DR have been published for diagnosis or prognosis, both of which involve predicting a label based on input data (AG, 2019).
Several techniques have been used in DR, including CNN, autoencoders (AEs), recurrent neural networks (RNNs), deep belief networks (DBNs). Despite several techniques, CNN found to be most suitable for imaging data as it learns to perform its task through repetition and self-correction (Browne & Ghidary, 2003; Schmidhuber, 2015; LeCun et al., 2015). Recently studies have applied CNN in DL in the detection of referable diabetic retinopathy (Abràmoff et al., 2018; Ting et al., 2017a; Gulshan et al., 2016; Abràmoff et al., 2016; Gargeya & Leng, 2017), glaucoma suspect (Ting et al., 2017b; Li et al., 2018), age-related macular degeneration (Ting et al., 2017b; Burlina et al., 2017; Grassmann et al., 2018) and retinopathy of prematurity (Brown et al., 2018). Gulshan et al. (Gulshan et al., 2016) developed CNN based DL system using 128175 macula-centred graded by a panel of ophthalmologists and 10000 images retrieved from two publicly available databases (EyePACS-1 and Messidor-2). Authors in this study reported 97.5% sensitivity and 93.4% specificity in the EYEPACS-1 while 96.1% sensitivity and 93.9% specificity for Messidor-2.
Gargeya and Leng (2017) developed diagnostic technology using deep convolutional neural networks (based on the principles of deep residual learning) to automate DR screening by processing color fundus images (75,137 images from the EyePACS public data), and classify as healthy or having DR. The model was tested using the public MESSIDOR 2 and E-Optha database for external validation. The developed model achieved 94% and 98% sensitivity and specificity with 97% accuracy. Kermany et al. (2018) utilized transfer learning to classify age-related macular degeneration and diabetic macular oedema. The author had reported an accuracy of 96.6%, with a sensitivity of 97.8%, a specificity of 97.4%. (de la Torre et al., 2019) used receptive field DL classifier for detection of the most severe case of DR. The model was trained with 75,550 images. The model surpassed human expert capabilities, reaching the performance of 90% of sensitivity and specificity in test sets of about 10,000 images of patients.
Earlier to train a DL algorithm, there is a requirement for human labeling of a reference training set. However, the study by Medeiros et al. (2019) used spectral-domain (SD) OCT data to train a DL algorithm to quantify glaucomatous structural damage on optic disc photographs. The study used residual deep CNN to train and assess optic disc photographs and predict SD-OCT average RNFL thickness. The author had reported 94% specificity and sensitivity in discriminating glaucomatous from healthy samples with the DL predicted and actual SD-OCT. On the other hand, (Krause et al., 2018) trained CNN algorithm (Ensemble, hyper-parameter using a Gaussian process bandit algorithm) using individual graders including US board-certified ophthalmologists and retinal specialists. The kappa ranged from 82% to 91% accuracy in comparison to individual retinal specialists.
But it is possible to prevent or delay if a high-risk group are identified at the right time and treated with the right approach. Machine learning algorithms do wonders where it helps to identify high risk population.
Contact us for more information about how we can help you to develop CER report.





Comment here